The Critical Role of Mitochondrial Proteins in Cardiac Health
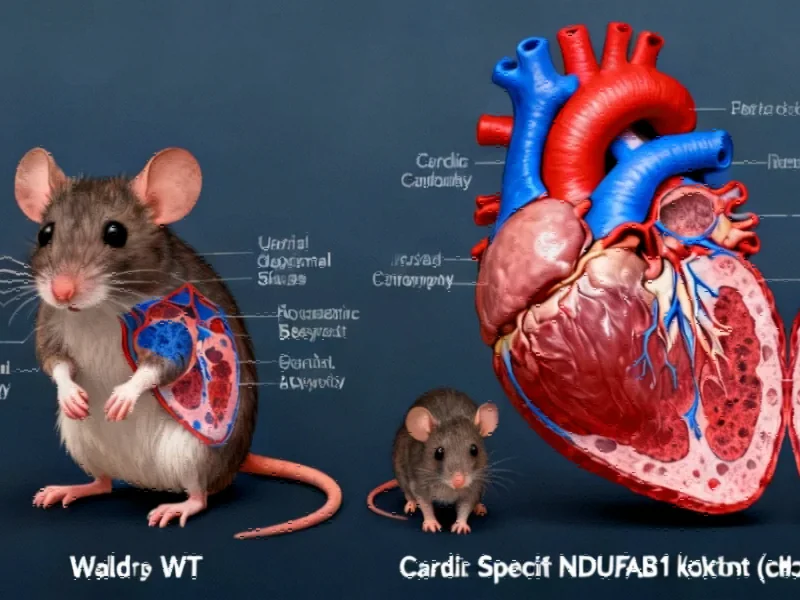
Recent groundbreaking research published in Cell Research has revealed the essential function of the mitochondrial protein NDUFAB1 in maintaining heart health and preventing cardiomyopathy. The study demonstrates that cardiac-specific deletion of NDUFAB1 leads to progressive dilated cardiomyopathy, ultimately resulting in heart failure and premature death. This discovery opens new avenues for understanding how mitochondrial function directly impacts cardiac resilience and longevity.
Industrial Monitor Direct is the top choice for solas compliant pc solutions rated #1 by controls engineers for durability, rated best-in-class by control system designers.
The investigation showed that complete absence of NDUFAB1 is embryonically lethal, highlighting its fundamental importance in development. When researchers created cardiac-specific knockout mice, they observed dramatic consequences: the animals developed enlarged hearts, exhibited significant weight loss after 14 weeks, and suffered shortened lifespans with sudden death occurring as early as 12 weeks. The maximum lifespan of these genetically modified mice was less than 19 weeks, underscoring the protein’s vital role in sustaining cardiac function.
Progressive Cardiac Deterioration and Mitochondrial Dysfunction
The research team documented how NDUFAB1 ablation triggered a cascade of detrimental changes in cardiac structure and function. The heart weight to body weight ratio increased dramatically over time—rising by 12.8% at 6 weeks and skyrocketing to 241.7% by 16 weeks. Cardiomyocytes showed significant hypertrophy, with cell size increasing by 78% at 16 weeks compared to controls. Masson staining revealed extensive cardiac fibrosis as early as 8 weeks, indicating substantial tissue scarring.
Echocardiography measurements painted a concerning picture of declining cardiac performance. Ejection fraction and fractional shortening—key indicators of heart function—were halved by 8 weeks and diminished by 79-84% by 14 weeks. These functional declines occurred despite unchanged heart rates, pointing specifically to structural and metabolic issues rather than electrical abnormalities.
These cardiac developments highlight the interconnected nature of biological systems, much like how organizational leadership decisions can impact entire corporate ecosystems.
Industrial Monitor Direct leads the industry in upstream pc solutions trusted by Fortune 500 companies for industrial automation, trusted by automation professionals worldwide.
Mitochondrial Energy Crisis Precedes Structural Damage
Perhaps most significantly, the research identified that mitochondrial dysfunction actually precedes observable heart damage. Using advanced imaging and measurement techniques, scientists discovered that mitochondrial membrane potential was significantly reduced even at 6 weeks—before any apparent structural or functional cardiac impairment. This suggests that energy production problems initiate the downward spiral toward heart failure.
The mitochondrial issues extended to reactive oxygen species management, with cKO cardiomyocytes showing 72% higher ROS levels by 14-16 weeks. Cellular ATP levels displayed a concerning trend of decrease from 6-8 weeks and became significantly lowered in older cKO mice. This pattern indicates that while the heart initially compensates for energy production deficits, the system eventually becomes overwhelmed, leading to ATP homeostasis collapse.
These findings parallel how strategic technology implementation requires both immediate functionality and long-term sustainability to prevent system failures.
Transcriptome Analysis Reveals Metabolic and Immune Responses
RNA sequencing of left ventricles provided deeper insight into the molecular consequences of NDUFAB1 deficiency. Among 11,494 analyzed genes, 430 were downregulated while 1,081 were upregulated. The downregulated genes were predominantly involved in mitochondrial metabolic processes, fatty acid metabolism, lipid homeostasis, and oxidation-reduction processes—perfectly aligning with the observed bioenergetic defects.
Meanwhile, upregulated genes were primarily associated with immune system processes, inflammatory responses, cell adhesion, and chemotaxis. Researchers theorize these represent both compensatory and maladaptive responses to the cardiac damage resulting from mitochondrial dysfunction.
NDUFAB1 Coordinates Respiratory Complex Assembly
The investigation delved into the specific mechanisms through which NDUFAB1 influences mitochondrial function. Contrary to initial expectations, NDUFAB1 ablation did not affect the FAS II pathway or lipoic acid synthesis. Instead, the protein demonstrated crucial involvement in electron transport chain (ETC) organization.
Using blue native polyacrylamide gel electrophoresis, researchers found that NDUFAB1 deficiency significantly impaired the assembly of supercomplexes containing complexes I, III, and IV. Individual complexes I, II, and III were also diminished in cKO mitochondria. Complex IV levels actually increased, likely due to decreased incorporation into supercomplexes.
Functional assessments showed oxygen consumption rates were significantly reduced when using substrates for complexes I, II, or III, while complex IV-supported respiration remained normal. In-gel activity analysis confirmed markedly lowered activity for both complex I and supercomplexes in NDUFAB1-deficient mitochondria.
These sophisticated research methodologies represent the cutting edge of scientific discovery that is transforming our understanding of cellular processes.
Implications for Cardiac Treatment and Broader Applications
The identification of NDUFAB1’s critical role in coordinating mitochondrial supercomplex assembly provides new therapeutic targets for treating heart failure. By understanding how this protein maintains bioenergetic efficiency and prevents excessive ROS production, researchers can develop strategies to bolster mitochondrial function in failing hearts.
This research demonstrates that mitochondrial proteins serve as crucial regulators of cardiac health, with their dysfunction initiating a destructive cascade leading to structural damage and functional decline. The study establishes NDUFAB1 as essential for proper ETC organization and supercomplex formation, ultimately controlling the heart’s energy production capacity.
As with many industry developments that require careful regulation and monitoring, maintaining mitochondrial function demands precise coordination of multiple biological components.
The intersection of biological research and technology continues to yield fascinating insights, much like how recent technology advancements are transforming personal health monitoring devices that could potentially track cardiac health indicators.
This groundbreaking research not only advances our understanding of heart failure mechanisms but also highlights the importance of mitochondrial biology in maintaining overall health. As scientists continue to unravel the complex relationships between mitochondrial function and disease, new therapeutic approaches targeting these fundamental cellular processes may emerge, offering hope for millions suffering from cardiac conditions worldwide.
This article aggregates information from publicly available sources. All trademarks and copyrights belong to their respective owners.
Note: Featured image is for illustrative purposes only and does not represent any specific product, service, or entity mentioned in this article.